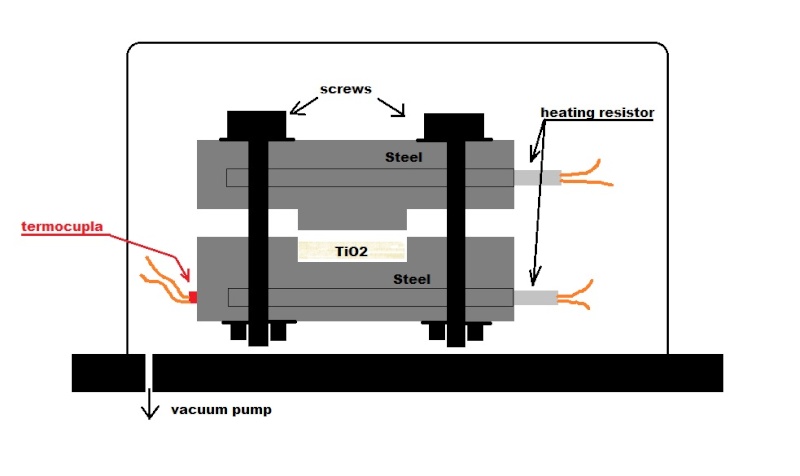
As I always state....I hope the translation goes correctly.
Sergio,
Here is a device that is essential in creating your own dielectrics:
http://www.keysight.com/en/pd-100000004 ... =US&lc=engIt will measure the Capacitance, the Dissippation Factor and most importantly...the K. I have in my shop a Leader LCR-745G. We have found it essential in testing the different configurations to establish the capacitance for the various dielectrics. This helps in determining the limiting resistance required so as not to overload the HV Power Supply.
http://www.testmart.com/webdata/mfr_pdf ... r-745g.pdfWhen we purchased the above unit, it was a bit more than what it is going for currently on ebay.
Test equipment, laboratory grade, is essential in recording your data for in the end, whatever you do, should be able to be recreated by others. It does get quite expensive.
Now, with that said, the motivations of the experimenter comes into play. Are you looking to satisfy your own personal curiosity or are you trying to establish a known phenomenon into some resemblance to becoming established science that can be engineered? If it is the later, it must be able to survive third party scrutiny and that is very difficult, thus, the need for meticulous measurements and that usually is where the price tag goes up.
Please....Please...do not take what I am saying here as discouragement. I state it as the reality and one must make a determination of their goals. For example, say you choose to just do the experiments and you find that you are getting good results. Then you take those results to someone in an attempt to show that you have done something and have attained good results. If you have not maintained meticulous measurements then you will not be taken seriously.
So, experiment away and I hope you attain promising results but always remember that if you discover something, you will need to start at the beginning and redo everything and make note of everything in a manner that will be acceptable to mainstream science.
Carry on!
Mikado